Carbon can exist in various kinds– called allotropes– offering it totally various homes from color to form to solidity. In a diamond every carbon atom is bonded to 4 surrounding carbons, whereas in graphite, every carbon atom is bonded to 3 surrounding carbons. The newly-synthesized particle is comprised of 48 carbon atoms in a rotating single/triple bond pattern. Called cyclo[48]carbon, it is steady enough for spectroscopic characterization in option at space temperature level.
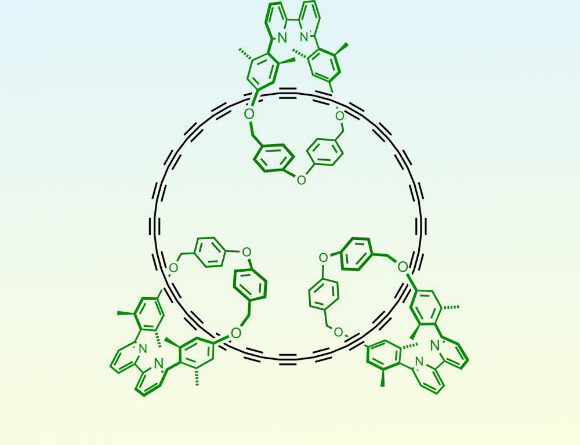
Chemical structure of the cyclo[48]carbon [4]catenan. Image credit: Harry Anderson.
Dr. Yueze Gao from the University of Oxford and associates manufactured the cyclo[48]carbon particle as a [4]catenane, i.e. with the C48 ring threaded through 3 other macrocycles.
These threaded macrocycles increase the stability of C48 by avoiding access to the secured cyclocarbon.
Formerly, molecular rings consisting simply of carbon atoms have actually just been studied in the gas stage or at really low temperature levels (4 to 10 K).
According to the group, cyclo[48]carbon is steady in service at 293 K (20 degrees Celsius).
This has actually been attained by utilizing threaded macrocycles, picking a big cyclocarbon with a low level of stress, and establishing moderate response conditions for the unmasking action in the response (where a precursor particle is changed into the end product).
“Achieving steady cyclocarbons in a vial at ambient conditions is a basic action,” Dr. Gao stated.
“This will make it much easier to study their reactivity and residential or commercial properties under regular lab conditions.”
The scientists then utilized mass spectrometry, NMR, UV-visible and Raman spectroscopy to identify the cyclocarbon catenane.
The observation of a single extreme 13C NMR resonance for all 48 sp1 carbon atoms shows that all of the carbons remain in comparable environments, which supplies strong proof for the cyclocarbon catenane structure.
“This accomplishment marks the conclusion of a long venture to manufacture cyclocarbon catenanes, based upon the hope that they may be steady adequate to study at space temperature level,” Professor Andersen stated.
The group’s work was released in the journal Science
_____
Yueze Gao et al2025. Solution-phase stabilization of a cyclocarbon by catenane development. Science 389 (6761 ): 708-710; doi: 10.1126/ science.ady6054
Find out more
As an Amazon Associate I earn from qualifying purchases.