 **Scientists Make Breasts in a Dish to Study Lactation**
**Scientists Make Breasts in a Dish to Study Lactation**
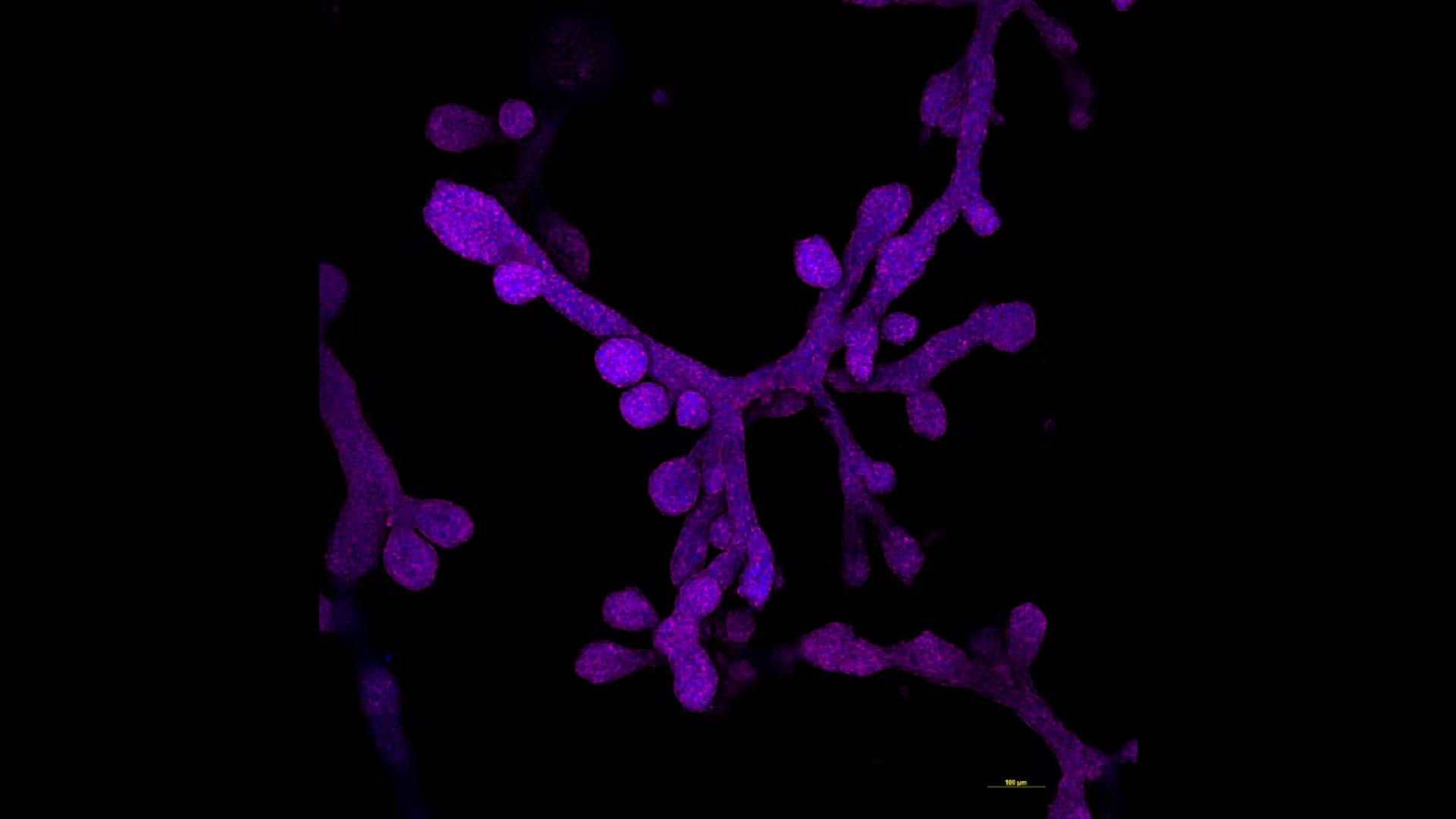
In a groundbreaking stride toward understanding the complexities of human lactation and the progression of breast cancer, scientists have successfully engineered mammary gland organoids that feature functional 3D milk ducts. These meticulously crafted mini-organs, or “organoids,” recreated in a laboratory setting, hold promise for unraveling some of the longstanding mysteries surrounding breast tissue development and disease.
Lactation is a finely tuned biological process vital to the nourishment of newborns, yet it remains a puzzling scientific topic. Traditional research methods have struggled to replicate the intricate architecture and dynamic behavior of mammary glands in vivo. However, with this innovative approach, researchers are now able to simulate these systems more accurately, shedding new light on the cellular and molecular mechanisms that govern milk production.
Breast cancer, a pressing global health concern, often originates in the milk ducts. By developing organoids with a realistic 3D ductal network, scientists aim to better understand how cancerous cells develop, proliferate, and spread within this microenvironment. This deeper understanding could pave the way for novel therapies targeted at early intervention and more personalized treatment options for patients.
The creation of these organoids involves the use of advanced stem cell technologies combined with 3D bioprinting and tissue engineering techniques. Scientists begin with pluripotent stem cells capable of differentiating into various cell types. Through a carefully directed differentiation process, these cells are coaxed into forming the complex structures that mimic real mammary glands. The achievement of functional milk ducts within these organoids marks a significant leap toward more accurate and effective breast tissue models.
Research utilizing these mammary gland organoids is already yielding valuable insights. For instance, studies have demonstrated how hormonal changes during pregnancy result in the expansion and regression of milk-producing cells, a process crucial for both lactation and breast tissue homeostasis. Additionally, by introducing cancerous mutations into these organoids, researchers can observe how malignant cells disrupt normal cellular architecture and invade surrounding tissues.
This innovative work is poised to transform our understanding of breast biology and oncology. It provides an invaluable platform for investigating the fundamental processes underlying lactation and breast cancer, representing a step forward in the quest for improved maternal health and cancer therapies.
As this research continues to evolve, the potential applications extend beyond the laboratory. Personalized medicine, where treatments are tailored to an individual’s unique biological characteristics, could benefit significantly. Drug testing and screening for breast cancer treatments could become more accurate, reducing reliance on animal models and expediting the development of effective therapies.
In conclusion, the creation of 3D mammary gland organoids marks a remarkable achievement in the fields of developmental biology and oncology. By providing researchers with a more realistic model of the human breast, this breakthrough paves the way for deepened understanding and innovative treatment approaches for both lactation-related conditions and breast cancer.
As an Amazon Associate I earn from qualifying purchases.

