As an Amazon Associate I earn from qualifying purchases.
drug increase–
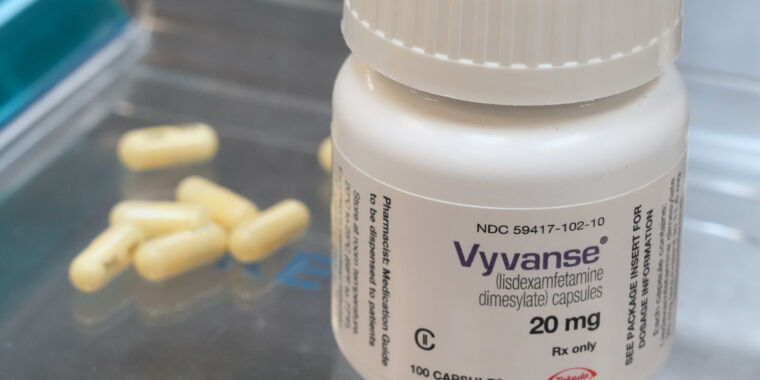
The DEA’s quota boost is for Vyvanse and its generic kinds.
Beth Mole
–
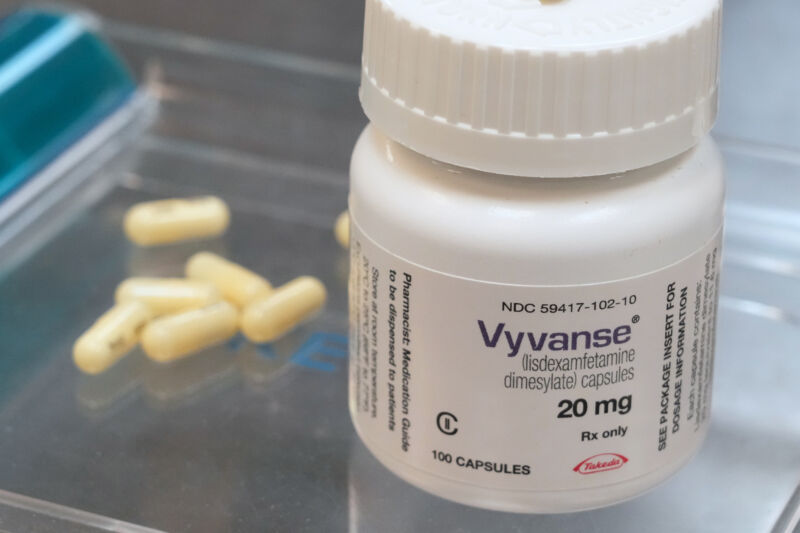
While products of Adderall and its generic variations are lastly recuperating after a yearslong lack, the Drug Enforcement Administration is now working to suppress the brief supply of another drug for attention-deficit/hyperactivity condition: Vyvanse (lisdexamfetamine) and its generic variations.
Today, the DEA stated it will increase the enabled production quantity of lisdexamfetamine by approximately 23.5 percent, increasing the existing 26,500 kg quota by 6,236 kg, for a brand-new overall of 32,736 kg. The DEA likewise permitted a matching boost in d-amphetamine, which is required for production of lisdexamfetamine.
“These modifications are required to guarantee that the United States has an appropriate and undisturbed supply of lisdexamfetamine to fulfill genuine client requires both locally and internationally,” the DEA stated.
Quotas
Much Like Adderall (amphetamine/dextroamphetamine salts), Vyvanse (lisdexamfetamine) is an amphetamine-class stimulant categorized by the DEA as a Schedule II drug. The DEA manages its production levels to guarantee need is fulfilled while avoiding excess supply that might discover its method to the black market. The administration does this by setting an “aggregate production quota”– which is what the DEA changed for lisdexamfetamine today– and administering concealed allocations to drug makers.
While different aspects have actually added to the lacks of ADHD medications, some medical and market groups have actually positioned blame on the DEA’s quota system for ignoring need and choking supply. The Adderall lack started in 2022 following a labor lack on the item’s production line at Teva, Adderall’s maker. While that production snag was dealt with, prescription rates increased substantially, in part due to increased awareness of ADHD, expanding medical diagnosis requirements, and a boost in gain access to with the increase of telehealth services, which expanded throughout the COVID-19 pandemic. In a report previously this year, the American Society of Health-System Pharmacists indicated the DEA’s quotas, stating they’re “intensifying” lacks.
In an August 2023 joint letter, the DEA and the FDA reacted to such criticism, recommending that the quotas aren’t to blame. Rather, it’s that some makers are not consuming their allocation of regulated drugs.
“Based on DEA’s internal analysis of stock, production, and sales information sent by makers of amphetamine items [which include the two ADHD drugs]makers just offered around 70 percent of their designated quota for the year, and there were around 1 billion more dosages that they might have produced however did not make or deliver. Information for 2023 up until now reveal a comparable pattern,” the FDA and DEA composed.
The FDA and DEA stated they would deal with producers to guarantee they would increase production of drugs in brief supply or relinquish their staying allocations.
Vyvanse scarcity
A likewise made complex scenario is seen with the existing shortage of Vyvanse and its generics. The DEA raised the quota after prodding from the Food and Drug Administration. In July, the FDA sent out the DEA a letter asking for a quota boost. The scarcity had in fact started in June 2023. At that time, Vyvanse’s maker, Takeda, stated that a “production hold-up intensified by increased need” had actually resulted in low stock.
In August 2023, the FDA authorized several generic variations of Vyvanse after Takeda’s patent exclusivity ended, raising hopes that the scarcity would reduce with the injection of brand-new generics. Supply issues have actually continued. In November, the Association for Accessible Medicines, which represents generic drugmakers, sent out a letter to the DEA stating that generic makers weren’t able to get adequate basic material to “introduce their items at complete business scale,” due to the fact that the quotas were standing in the method, according to reporting by Bloomberg.
FiercePharma reported another prospective element raised by legislators and market watchers. Those observers kept in mind of the timing of Takeda’s “production hold-ups” simply months before generics got in the marketplace. With the considerably thinner earnings margin of generic and off-patent drugs, there’s issue that makers might de-prioritize production.
Last, the DEA flagged yet another consider the supply chain: exports to foreign markets. While the FDA approximated a 6 percent boost in the domestic requirement for lisdexamfetamine in between 2023 and 2024, the DEA’s export information revealed a 34 percent boost in exports of lisdexamfetamine in between 2022 and 2023, with expectations that exports would continue to increase this year and beyond. The present 23.5 percent quota boost for lisdexamfetamine is just partially for domestic production. Just a quarter of the 6,236 kg is meant for the United States. Of the increased allocation, 1,558 kg is for domestic drug production, while the other 4,678 kg addresses increases in foreign need, the DEA stated.
As an Amazon Associate I earn from qualifying purchases.