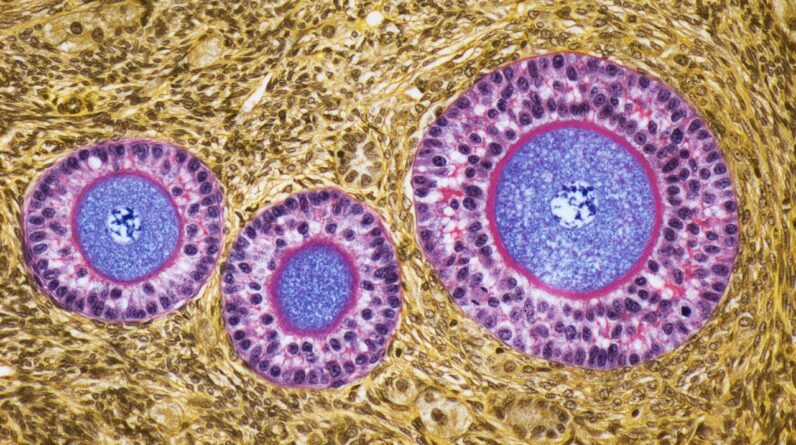
(Image credit: STEVE GSCHMEISSNER/SCIENCE PHOTO LIBRARY through Getty Images)
Researchers have actually taken an essential action towards comprehending why human eggs grow more susceptible to chromosomal mistakes as they age and whether that decrease might be prevented one day.
The research study, released in November in the journal Nature Agingpresents a brand-new tool that makes it possible for researchers to reproduce modifications seen in eggs throughout the aging procedure. The strategy, which utilizes mouse egg cells, does not need scientists to wait on the mice to age or to gather aged human eggs for research study, and it allows them to no in on various forces that may add to an egg’s decrease.
This research study remains in its early days, however ultimately, the research study authors hope it might assist extend the reproductive windows of ladies who prepare to have kids later on in life.
“Female reproductive aging is a major source of inequity,” stated senior research study author Binyam Mogessiean assistant teacher at the Yale University School of Medicine. “Women have to make choices men don’t have to make” when it pertains to weighing when to begin a household. Significantly, the rate of under-30 births is now trending down as over-30 births trend up in the U.S. Simply put, more ladies are having infants at older ages, when the rate of chromosomal irregularities starts to increase.
“Even if we can extend this reproductive window by three years, it would be so consequential to the lives of so many people,” Mogessie informed Live Science.
A design of aging eggsLadies are born with all the egg cells they’ll ever bring, and gradually, those eggs are launched through the menstruation. Eggs that have yet to be launched hang out in the ovaries, where numerous will remain for years.
Get the world’s most remarkable discoveries provided directly to your inbox.
Around age 30, this waiting egg supply reveals a sharp uptick in aneuploidy danger, implying the eggs are most likely to bring an irregular variety of chromosomes– either basically than 46. Research studies reveal that the threat of egg aneuploidy grows nearly greatly after age 35and after that leaps once again at 40 and at 45. These chromosomal irregularities can add to infertility and pregnancy loss in females, in addition to congenital diseases in kids, a few of which can trigger serious special needs or death
Researchers are still uncertain why aneuploidy danger increases a lot with age. “The leading theory is that the forces that hold these chromosomes together, before they are separated at fertilization, those forces are failing progressively with age,” Mogessie stated.
At different points in an egg’s cell cycle, each of its chromosomes consists of 2 “sister chromatids” held together by molecular glue, and those sis later on get pulled apart. That glue is understood to compromise with age and hence result in chromatid separation problems that add to aneuploidy. That does not inform the entire story; it does not discuss why we see a sharp increase in chromosomal mistakes beginning around age 30, Mogessie stated.
To examine this secret, the scientists established a speculative setup to activate “aging-like” modifications in eggs and view how the eggs altered later, utilizing high-resolution time-lapse microscopy. A crucial part of the design was making use of the gene-editing system CRISPR to modify a vital element of the molecular glue that holds chromosomes together: a protein called REC8.
This tweak included a switch to REC8, and when that switch was toggled “on,” the protein would break down. Utilizing this system, the researchers might firmly manage the degree of REC8 breakdown in an egg, mimicing what would take place naturally throughout aging.
“In animals, it can take years; in humans, it can take decades for these processes to arise,” Mogessie stated. The brand-new method “allows us to do this within 60 to 90 minutes.”
Formerly, Mogessie and partners had utilized antibodies to tinker REC8 in a comparable method, however this included injecting the antibodies into fragile egg cells– a picky and labor-intensive procedure– and the degree of destruction was tough to manage, Mihalas kept in mind. Some advantages of the brand-new system are that you prevent injecting the eggs and can tune REC8 levels far more exactly. “It is quite elegant,” she stated.
Leading the way to future treatmentsThe group showed that degrading REC8 to differing degrees caused mistakes in chromosome splitting and to aneuploidy, as you ‘d anticipate to see in naturally aged eggs. This likewise allowed them to determine a particular limit of REC8 loss at which the rate of mistakes all of a sudden increased.
While the loss of REC8 might set off these problems, researchers understand that eggs decrease in extra methods with age. To design this, the group messed with other proteins included in holding chromosomes together, as well as with filaments that pull them apart when the time is. These perturbations improved the rate of chromosomal mistakes beyond what was seen with REC8 loss alone.
Taken together, these outcomes recommend that the breakdown of chromosomes’ molecular glue most likely sets the phase for aneuploidy. The abrupt spike seen in individuals in their 30s and 40s most likely stems from the “synergistic failure” of numerous parts of this chromosome-separating equipment, the group stated.
More research study is required to totally comprehend the effect of aging on eggs, however the brand-new design ought to make it possible for such work to be done. “The mouse model provides consistency,” Mihalas kept in mind. Provided the ethical obstacles and restrictions of dealing with human eggs, “it’s the best model we have,” Mihalas included.
In the long run, the design might be utilized to evaluate for and check the impacts of prospective treatments. There might be a method to reverse the clock and assistance eggs to dependably share less chromosomal mistakes, as they would have at more youthful ages.
“It really does set the scene for preventive measures aimed at improving the quality of eggs, at least in an IVF [in vitro fertilization] clinic setting,” Mogessie stated. “I think that would have a huge impact.”
This short article is for educational functions just and is not implied to provide medical suggestions.
Nicoletta Lanese is the health channel editor at Live Science and was formerly a news editor and personnel author at the website. She holds a graduate certificate in science interaction from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has actually appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, to name a few outlets. Based in NYC, she likewise stays greatly associated with dance and carries out in regional choreographers’ work.
Learn more
As an Amazon Associate I earn from qualifying purchases.