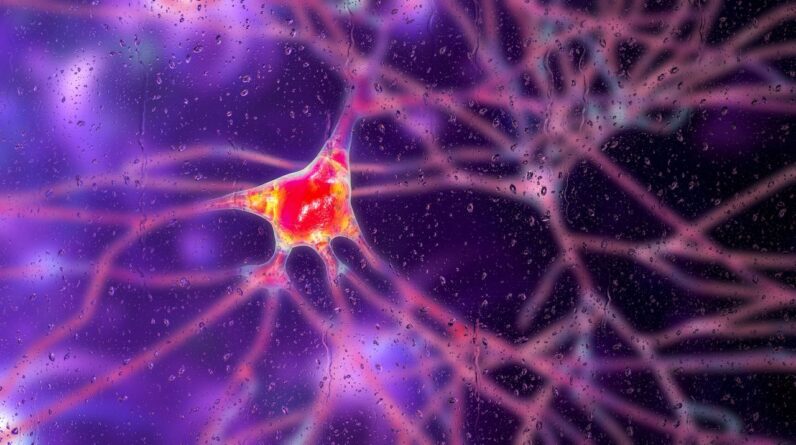
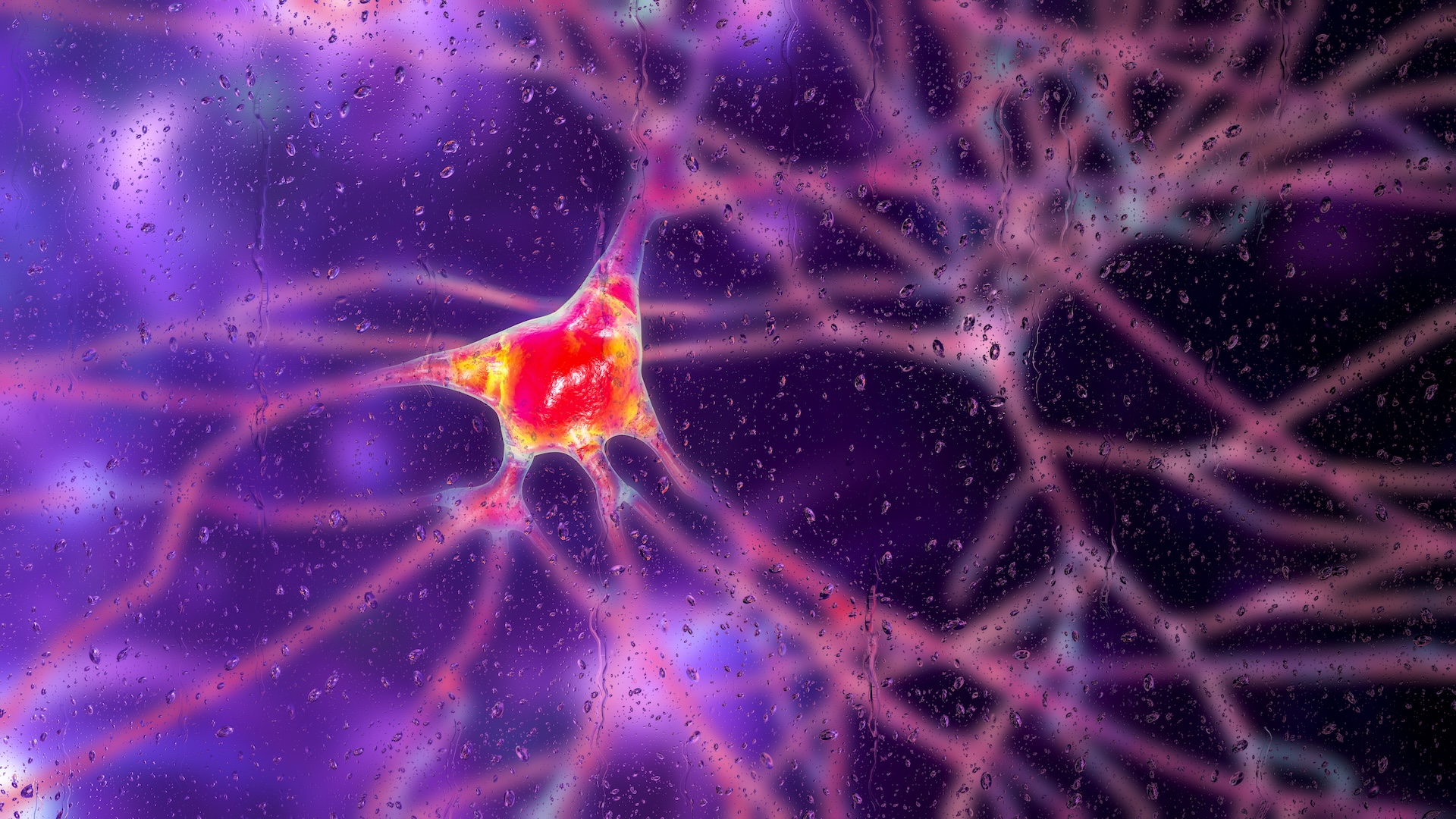
Huntington’s illness is an inheritable condition that triggers brain cells to pass away. A brand-new gene treatment might assist slow the illness’s development, trial information recommend.
( Image credit: KATERYNA KON/SCIENCE PHOTO LIBRARY by means of Getty Images)
In a groundbreaking initially, a gene treatment in scientific trials has actually slowed the development of Huntington’s illness, an uncommon congenital disease in which poisonous littles protein cause brain cells to breakdown and pass away.
To date, authorized treatments for Huntington’s illness objective to handle its signswhich frequently emerge in an individual’s 30s or 40s. The progressive condition hurts and eliminates crucial nerve cells associated with managing state of mind, cognition and motor control. Numerous drugs can assist to balance out the anxiety, hallucinations and improperly collaborated motions that emerge from that damage.
Now, in trial results shared Wednesday (Sept. 24), researchers revealed that a brand-new gene treatment called AMT-130 appears to slow the illness’s development– marking a very first for the field.
“These groundbreaking data are the most convincing evidence in the field to date and underscore the disease-modifying effect in Huntington’s disease, where an urgent need persists,” Dr. Sarah Tabrizithe lead clinical consultant on the trial and the director of the University College London (UCL) Huntington’s Disease Centre, stated in a declaration “For patients, AMT-130 has the potential to preserve daily function, keep them in work longer, and meaningfully slow disease progression.”
Huntington’s illness, approximated to impact about 1 in every 20,000 to 10,000 individuals in the U.S., is triggered by anomalies in a gene called HTTwhich brings guidelines for a protein referred to as huntingtin. The protein is discovered in lots of tissues throughout the body, however its amounts are greatest in the brain. The function of the protein in cells isn’t completely comprehended, Its proposed tasks consist of fixing damage to DNA and carrying products within cells.
There’s a part of the HTT gene in which 3 letters– CAG– in its DNA code repeat about 10 to 35 times, depending upon the individual. In individuals with Huntington’s illness the repeating ends up being severe, with CAG appearing 36 to over 120 times. Individuals with 40 or more repeats almost constantly establish the illness, while those with 36 to 39 have a lower danger. The repeats lead to cells making a too-long variation of the huntingtin protein, which then gets damaged apart into smaller sized, poisonous pieces that build up inside brain cells.
Get the world’s most interesting discoveries provided directly to your inbox.
The gene treatment AMT-130, established by the trial’s sponsor uniQure, works by silencing the HTT gene– both healthy and mutant variations. Significantly, gene treatments aren’t usually 100% effective, suggesting the treatment would not impact every copy of HTT in the targeted tissue; so instead of removing the gene’s activity, it turns it down considerably.
To do so, the treatment presents a brand-new gene into cells in 2 parts of the brain struck hard by Huntington’s: the putamen and caudate nucleus. The gene itself brings guidelines for a microRNAa kind of particle that manages gene activity. In this case, the microRNA thwarts the procedure by which the HTT gene’s code gets equated into proteins. It acquires messenger RNA (mRNA) in cells, which would typically communicate the HTT’s plans out to protein-building factories in the cell.
The AMT-130 treatment is provided into the body inside a safe infectionwhich works as a delivery van for the microRNA. (These kinds of infections are frequently utilized in gene treatment) Getting the treatment into the brain needs a complex surgical treatment, throughout which medical professionals utilize MRI to assist small catheters into the proper areas in the organ. The treatment is given up one dosage, so just one surgical treatment is required to administer it.
In the trial, 29 clients got this brand-new treatment, with 17 getting a high dosage and 12 getting a low dosage. Twelve clients from each group then had 3 years of follow-ups that were consisted of in this brand-new analysis.
The cured clients were compared versus an associate of individuals with Huntington’s who got just basic care and are being followed in a long-lasting research study called Enroll-HD. The trial runners utilized a basic ranking scale for Huntington’s illness development to track clients and compare them to one another. They likewise determined clients’ levels of neurofilament light protein (NfL), which appears in the fluid surrounding the spine when nerve cells are hurt.
The trial results revealed that, at the three-year mark, clients provided the high dosage of AMT-130 had 75% less illness development compared to the mate provided basic treatment. The high-dose group likewise revealed a decrease in typical NfL levels over that timeframe, recommending a decrease in the degree of neuronal damage being wrought. Usually, the protein’s levels would increase by about 20% to 30% over 3 years.
“AMT-130 was generally well-tolerated, with a manageable safety profile at both doses,” the declaration notes. “The most common adverse events [side effects] in the treatment groups were related to the administration procedure, which all resolved.”
“My patients in the trial are stable over time in a way I’m not used to seeing in Huntington’s disease,” Dr. Ed Wildprimary detective of the trial website at the UCL Huntington’s Disease Centre, stated in the declaration. “One of them is my only medically-retired Huntington’s disease patient who has been able to go back to work.”
He included that “trial results come through in numbers and graphs, but behind each datapoint is an incredible patient who volunteered to undergo major neurosurgery to be treated with the first gene therapy we’ve ever tested in Huntington’s disease. That is an extraordinary act of bravery for the benefit of humanity.”
According to the declarations launched by uniQure and UCL, the business prepares to send an approval application to the U.S. Food and Drug Administration (FDA) early next year, with applications in Europe to follow. AMT-130 has actually currently been given Advancement Therapy classification and Regenerative Medicine Advanced Therapy classification by the U.S. FDA, which both signal that regulators feel the treatment holds excellent pledge to deal with clients with an unmet medical requirement.
This post is for informative functions just and is not indicated to provide medical suggestions.
Nicoletta Lanese is the health channel editor at Live Science and was formerly a news editor and personnel author at the website. She holds a graduate certificate in science interaction from UC Santa Cruz and degrees in neuroscience and dance from the University of Florida. Her work has actually appeared in The Scientist, Science News, the Mercury News, Mongabay and Stanford Medicine Magazine, to name a few outlets. Based in NYC, she likewise stays greatly associated with dance and carries out in regional choreographers’ work.
Learn more
As an Amazon Associate I earn from qualifying purchases.