(Image credit: CHDENK, CC BY-SA 4.0, through Wikimedia Commons)
Mitochondria have actually mainly been called the energy-producing elements of cells. Researchers are significantly finding that these little organelles do a lot more than simply power cells. They are likewise associated with immune functions such as managing swelling controling cell death and reacting to infections
Research study from my associates and I exposed that mitochondria play another essential function in your immune reaction: picking up bacterial activity and assisting neutrophils, a kind of leukocyte, trap and eliminate them.
For the previous 16 years, my research study has actually focused on comprehending the choices immune cells make throughout infection and how the breakdown of these decision-making procedures trigger illness. My laboratory’s current findings clarify why individuals with autoimmune illness such as lupus might have a hard time to combat infections, exposing a possible link in between inefficient mitochondria and deteriorated immune defenses
The body immune system’s ace in the holes
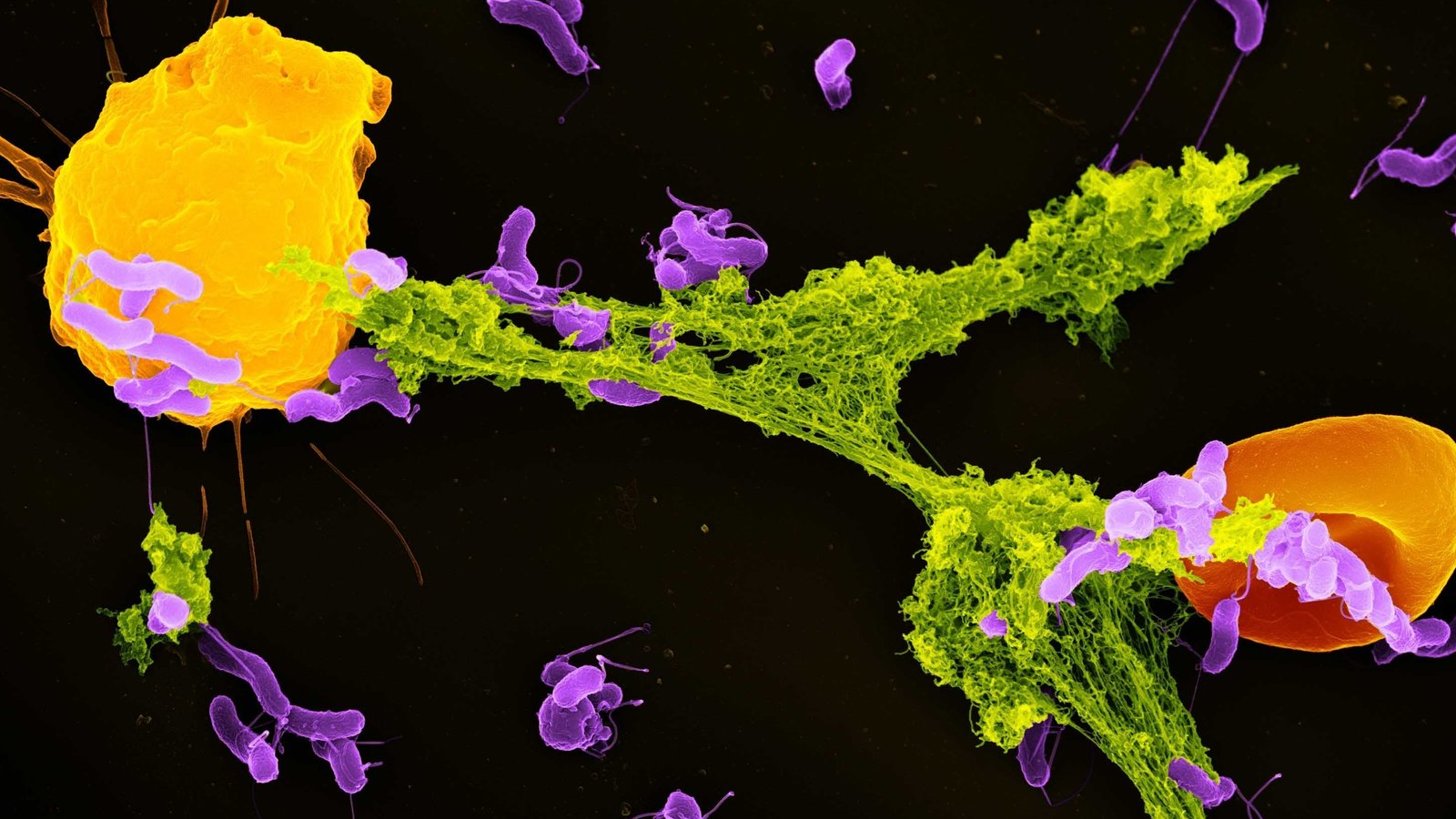
Neutrophils are the most plentiful kind of immune cell and function as the body immune system’s Responders. Among their crucial defense reaction is launching neutrophil extracellular traps, or NETs — weblike structures made up of DNA and antimicrobial proteins. These sticky NETs trap and reduce the effects of getting into microorganisms, avoiding their spread in the body.
Till just recently, researchers thought that NET development was mostly set off by cellular tension and damage. Our research study discovered that mitochondria can discover a particular bacterial by-product– lactate– and utilize that signal to start NET development.
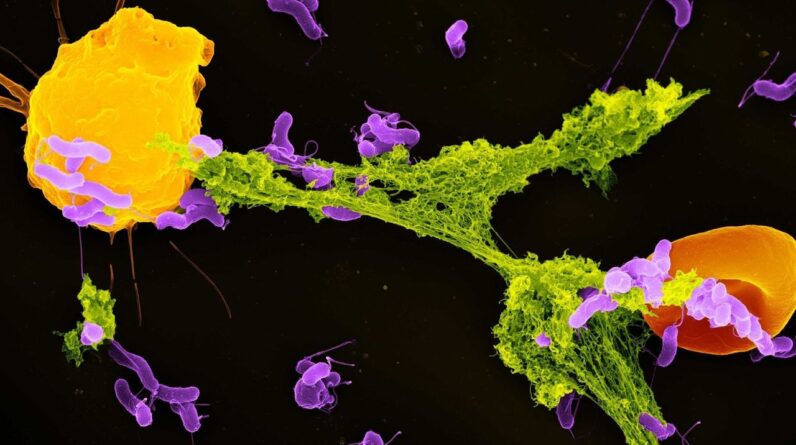
When mitochondria (envisioned above) breakdown, it might deteriorate the body’s immune defenses. (Image credit: OpenStax, CC BY-SA)
Lactate is frequently related to muscle tiredness in individualsIn the context of bacterial infectionsit plays a various function. Lots of germs launch lactate as part of their own energy production. My group discovered that as soon as germs are swallowed up by a compartment of the cell called the phagosomeneutrophils can pick up the existence of this lactate.
Related: 8 children spared from possibly lethal acquired illness through brand-new IVF ‘mitochondrial contribution’ trial
Get the world’s most remarkable discoveries provided directly to your inbox.
Inside the phagosome, this lactate interacts to the neutrophil that germs exist which the anti-bacterial procedures are not enough to eliminate these pathogens. When the mitochondria in neutrophil cells find this lactate, they start signaling for the cell to eliminate the NETs that have actually allured germs. As soon as the germs are launched outside the cell, other immune cells can eliminate them.
When we obstructed the mitochondria’s capability to sense lactate, neutrophils stopped working to produce NETs efficientlyThis indicated germs were most likely to leave capture and multiply, demonstrating how essential this system is to immune defense. This procedure highlights a detailed discussion in between the germs’s metabolic process and the host cell’s energy equipment.
What makes this finding unexpected is that the mitochondria within cells have the ability to discover germs caught in phagosomes, although the microorganisms are confined in a different area. In some way, mitochondrial sensing units can get hints from within these compartments– a remarkable accomplishment of cellular coordination.
Targeting mitochondria to combat infections
Our research study belongs to a growing field called immunometabolismwhich checks out how metabolic process and immune function are deeply linked. Instead of seeing cellular metabolic process as strictly a way to create energy, scientists are now acknowledging it as a main motorist of immune choices.
Mitochondria sit at the heart of this interaction. Their capability to sense, react to and even form the metabolic environment of a cell provides a vital function in figuring out how and when immune reactions are released.
Our findings supply an essential factor why clients with a persistent autoimmune illness called systemic lupus erythematosus frequently experience persistent infections. Mitochondria in the neutrophils of lupus clients stop working to sense bacterial lactate effectively. As an outcome, NET production was substantially decreased. This mitochondrial dysfunction might discuss why lupus clients are more susceptible to bacterial infections– despite the fact that their body immune systems are continuously triggered due to the illness.
This observation indicate mitochondria’s main function in stabilizing immune reactions. It links 2 relatively unassociated concerns: immune overactivity, as seen in lupus, and immune weak point like increased vulnerability to infection. When mitochondria work properly, they assist neutrophils install an efficient, targeted attack on germs. When mitochondria are impaired, this system breaks down.
Our discovery that mitochondria can pick up bacterial lactate to set off NET development opens brand-new possibilities for dealing with infections. Drugs that improve mitochondrial noticing might improve NET production in individuals with weakened immune systems. On the other side, for conditions where NETs add to tissue damage– such as in serious COVID-19 or autoimmune illness– it may be advantageous to restrict this reaction.
In addition, our research study raises the concern of whether other immune cells utilize comparable systems to notice microbial metabolites, and whether other bacterial by-products may work as immune signals. Comprehending these paths in more information might result in brand-new treatments that regulate immune actions more specifically, lowering civilian casualties while maintaining antimicrobial defenses.
Mitochondria are not simply the powerhouses of the cell– they are the body immune system’s watchtowers, alert to even the faintest metabolic signals of bacterial intruders. As scientists’ understanding of their functions broadens, so too does our gratitude for the intricacy– and flexibility– of our cellular defenses.
This edited short article is republished from The Conversation under a Creative Commons license. Check out the initial post
The objective of the Monteith lab is to check out the fragile balance existing in between metabolic and inflammatory procedures, and how perturbations in this stability promote illness. It leverages knowledge throughout several illness with the hopes of establishing impactful immunological discoveries and equating our findings to enhance human health.
Learn more
As an Amazon Associate I earn from qualifying purchases.