Chemists at the Leibniz Institute for Tropospheric Research have actually supplied speculative proof that sulfurous acid (H23when formed in the gas stage, is kinetically steady sufficient to enable its characterization and subsequent responses.
In the gas stage, if as soon as formed, sulfurous acid reveals a particular kinetic stability with an approximated life time of a minimum of one 2nd for climatic water vapor conditions. Image credit: Berndt et aldoi: 10.1002/ anie.202405572.
Sulfurous acid has the formula H23 and the molecular weight of 82.075 g/mol.
Understood as sulfuric (IV) acid and thionic acid, this particle represents a hard-to-reach acid that has actually never ever been observed in liquid service.
Sulfurous acid has actually been identified in the gas stage in 1988 by the dissociative ionization of diethyl sulfite.
“The only speculative detection of sulfurous acid to date was accomplished by Helmut Schwarz’s group at TU Berlin in 1988 utilizing in-situ generation in a mass spectrometer,” stated Dr. Torsten Berndt and his associates at the Leibniz Institute for Tropospheric Research.
“An incredibly brief life time under vacuum conditions in the series of 10 split seconds and more was approximated.”
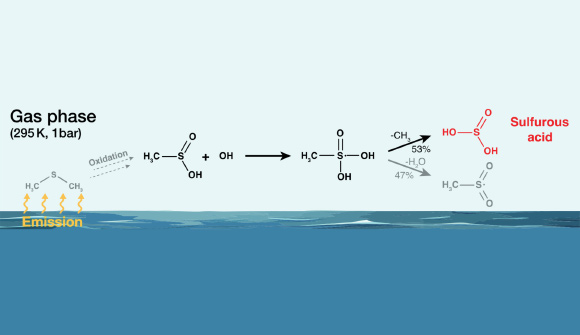
“Theoretical estimations recommended the development of H23 as a possible response item of the gas-phase response of OH radicals, which are formed in the troposphere mostly from ozone and water particles in the existence of UV radiation, with dimethyl sulfide (DMS).”
“DMS is generally produced by biological procedures in the sea and is the biggest biogenic sulfur source for the environment, producing around 30 million lots each year.”
The research study authors experimentally examined the possible response path to H23 beginning with the DMS.
The development of H23 in the gas stage was plainly shown in circulation reactors under climatic conditions.
“Under the speculative conditions, the sulfurous acid stayed steady for half a minute despite the humidity,” the scientists stated.
“Longer home times might not yet be examined with the existing speculative setup.”
“Therefore, H23 might likewise exist adequately enough time in the environment and have an impact on the chemical procedures.”
“The observed yield was even rather higher than in theory presumed.”
The associated design simulations revealed that around 8 million lots of H23 are formed worldwide every year.
“This path produces about 200 times more mass of H23 than the direct development of sulfuric acid (H24from dimethyl sulfide in the environment,” stated Dr. Andreas Tilgner and Dr. Erik Hoffmann, likewise from the Leibniz Institute for Tropospheric Research.
“The brand-new outcomes can add to a much better understanding of the climatic sulfur cycle.”
The group’s paper was released in the journal Angewandte Chemie
_____
Torsten Berndt et al2024. Gas-Phase Formation of Sulfurous Acid (H23in the Atmosphere. Angewandte Chemie 63 (30 ): e202405572; doi: 10.1002/ anie.202405572
As an Amazon Associate I earn from qualifying purchases.







