Ice at minus 10 degrees Celsius launches more iron from typical minerals than liquid water at 4 degrees Celsius, according to a group of scientists from Umeå University, the Institut des Sciences Chimiques de Rennes and CNRS. This discovery might assist discuss why lots of Arctic rivers are now turning rusty orange as permafrost defrosts in a warming environment.
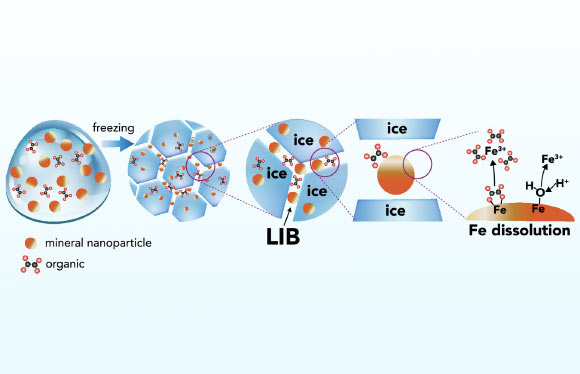
Schematic representation of iron mineral dissolution responses in ice. Image credit: Sebaaly et aldoi: 10.1073/ pnas.2507588122.
“It might sound counterproductive, however ice is not a passive frozen block, “stated Umeå University’s Professor Jean-François Boily.
” Freezing develops tiny pockets of liquid water in between ice crystals. “
“These imitate chemical reactors, where substances end up being focused and exceptionally acidic.”
“This indicates they can respond with iron minerals even at temperature levels as low as minus 30 degrees Celsius.”
To comprehend the procedure, Professor Boily and his coworkers studied goethite– a prevalent iron oxide mineral– together with a naturally taking place natural acid.
Utilizing sophisticated microscopy and experiments, they found that duplicated freeze-thaw cycles make iron liquify more effectively.
As the ice freezes and defrosts, natural substances that were formerly caught in the ice are launched, sustaining more chain reaction.
Salinity likewise plays an essential function: fresh and brackish water boost dissolution, while seawater can reduce it.
The findings use generally to acidic environments, such as mine drain websites, frozen dust in the environment, acid sulfate soils along the Baltic Sea coast, or in any acidic frozen environment where iron minerals engage with organics.
“As the environment warms, freeze-thaw cycles end up being more regular,” stated Angelo Pio Sebaaly, a doctoral trainee at Umeå University.
“Each cycle launches iron from soils and permafrost into the water. This can impact water quality and water communities throughout large locations.”
“The findings reveal that ice is not a passive storage medium, however an active gamer.”
“As freezing and thawing boost in polar and mountain areas, for the effect on environments and the natural biking of aspects might be substantial.”
The group’s paper was released on August 26, 2025 in the Procedures of the National Academy of Sciences
_____
Angelo P. Sebaaly et al2025. Ice as a kinetic and mechanistic chauffeur of oxalate-promoted iron oxyhydroxide dissolution. PNAS 122 (35 ): e2507588122; doi: 10.1073/ pnas.2507588122
Learn more
As an Amazon Associate I earn from qualifying purchases.