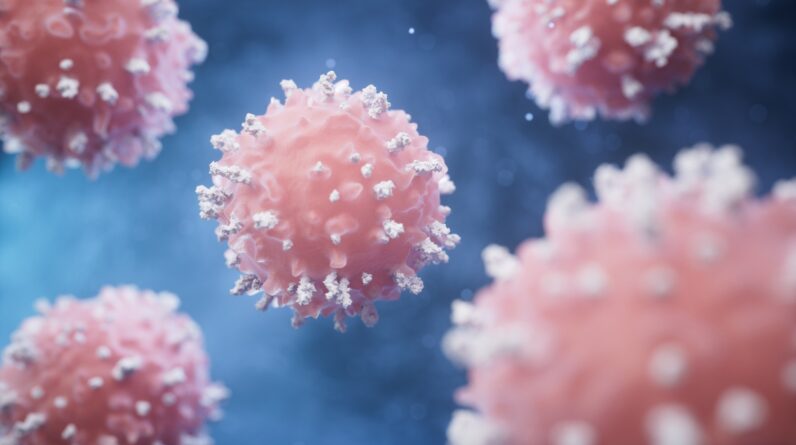
The scientists customized T cells, a kind of lymphocyte(revealed above), to safeguard pancreatic cells.
(Image credit: Jian Fan through Getty Images)
In an initially, researchers have actually developed immune cells that safeguard stem cell transplants from being turned down by the body– and they might sooner or later unlock for a treatment for diabetes.
The brand-new cells, which had the ability to safeguard insulin-producing cells transplanted into mice, are an early “proof-of-concept,” stated research study co-author Audrey Parent, an associate teacher at the University of California, San Francisco (UCSF)Diabetes.
If revealed to be safe and efficient in individuals, the designer cells might one day be utilized to secure transplanted tissues from attack, decreasing or getting rid of the requirement for drugs that reduce the body immune systemThat, in turn, might lead the way to a treatment for illness like type 1 diabetes.
In type 1 diabetes, immune cells, referred to as killer T cells, ruin pancreatic beta cells, that make insulin. Recently, researchers have actually gradually inched closer to changing damaged beta cells with brand-new cells originated from stem cells, which can be made to develop into any kind of cell in the body.
Related: ‘Like a reset button on a computer system’: Designer cells’reboot’body immune system in 3 various autoimmune illness
In June, for example, researchers reversed type 1 diabetes in an individual by reprogramming their fat cellswhile the Boston-based business Vertex Pharmaceuticals just recently released a critical, massive trial screening whether reprogrammed stem cells can remove the requirement for insulin in those with type 1 diabetes.
Before such stem-cell transplants can be commonly utilized, researchers require to resolve one huge issue: In type 1 diabetes, killer T cells have actually been trained to target beta cells and have actually currently damaged those cells when. The transplanted cells require security from this immune attack, so in the meantime, clients require strong drugs that reduce the body immune system. These drugs leave clients open to harmful infections and are poisonous to the kidneys and other organs.
Get the world’s most interesting discoveries provided directly to your inbox.
To navigate this issue, Parent and associates crafted T cells in the laboratory that secured the transplanted cells– referred to as a graft– from attack.
“We took an immune cell and changed the machinery inside it to make it a protective cell instead of a killer cell,” Moms and dad informed Live Science. “And then we targeted it to the graft.” Basically, the designer cells function as bodyguards.
The bodyguards no in on beta cells due to the fact that they acknowledge a particular protein, called CD19, that the scientists contributed to the beta cells. When the bodyguard cells get onto CD19, they then crank out a particle that hinders killer T cells.
The guards likewise make a protein that absorbs an inflammatory chemical that typically assists trigger killer T cells. This anti-inflammatory protein likewise informs the guards to reproduce, developing a favorable feedback loop that strengthens their ranks, Parent stated.
To evaluate their guards in a living organism, the scientists then took beta cells originated from stem cells and implanted them into mice. They then sent out killer T cells to assault the transplanted beta cells. In one group of mice, they likewise injected their designer cells to protect the transplants.
In the mice not provided designer cells, the killer cells rapidly eliminated all the beta cells. In the mice injected with designer cells, the transplants lived at least 35 days, and the mice were still producing insulin at that time, scientists reported in the research study, released Thursday (Dec. 5) in the journal Science
The outcomes reveal it is possible to engineer T cells that can secure transplanted tissue, Parent stated.
One obstacle is discovering a distinct protein target to trigger the designer cells, Parent stated, as the majority of prospective targets are discovered on cells in several locations in the body. That raises the possibilities that their designer cells will trigger in other places in the body, beyond the transplants. That might posture an issue if, for example, cells with the protein target end up being contaminated or malignant, however can’t be cleared due to the fact that they are being safeguarded by the guard cells. Transplant cells might be crafted to have a “kill” switch for those cases, however other cells in the body would not have this switch.
Follow-up work might resolve this issue. The group might craft a synthetic target that would be discovered just on the transplanted beta cells and no place else, research study co-author Wendell Lima biochemist and director of the UCSF Cell Design Institute, informed Live Science in an e-mail.
In a different research study, likewise released Thursday in ScienceLim and coworkers revealed that comparable designer T cells might target brain growth cells while leaving healthy brain cells alone. The cells might likewise provide anti-inflammatory chemicals to brain cells in mice with an illness comparable to several sclerosis.
Looking forward, the group is likewise thinking about seeing how this method works versus other autoimmune illness sustained by swelling, such as rheumatoid arthritis, in addition to Crohn’s illness and other inflammatory bowel illness, Lim included. It will be numerous years before these concepts can be checked in human beings, he stated.
“This work opens up a new avenue for treating inflammatory diseases in a targeted way,” he stated,” but lots of pieces need to be put together and tested to come up with effective therapies.”
Ever question why some individuals develop muscle more quickly than others or why freckles come out in the sunSend us your concerns about how the body works to community@livescience.com with the subject line “Health Desk Q,” and you might see your concern responded to on the site!
Tia is the handling editor and was formerly a senior author for Live Science. Her work has actually appeared in Scientific American, Wired.com and other outlets. She holds a master’s degree in bioengineering from the University of Washington, a graduate certificate in science composing from UC Santa Cruz and a bachelor’s degree in mechanical engineering from the University of Texas at Austin. Tia belonged to a group at the Milwaukee Journal Sentinel that released the Empty Cradles series on preterm births, which won several awards, consisting of the 2012 Casey Medal for Meritorious Journalism.
Many Popular
Find out more
As an Amazon Associate I earn from qualifying purchases.